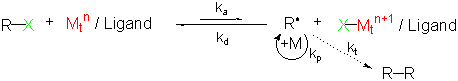
Atom Transfer Radical Polymerization (ATRP)
Atom transfer radical polymerization is a controlled/“living” polymerization based on the use of radical polymerization to convert monomer to polymer. Although many of the polymer types described have been prepared using other living polymerizations, researchers have been striving to develop a living radical polymerization for nearly 40 years. An alternative was sought because other types of living polymerizations are severely limited by many factors: only a small number of monomers can be used, the reactions are sensitive to moisture, and two or more monomers cannot be randomly copolymerized. Radical polymerization, in contrast, can polymerize hundreds of monomers, can copolymerize two or more monomers, and can be performed in water as emulsions or suspensions. Controlled/“living” radical polymerization promised to overcome these limitations and provide a method to maximize the potential of living polymerizations.
The Matyjaszewski research group was the first to develop a controlled/“living” polymerization that used a simple, inexpensive polymerization system. It is capable of polymerizing a wide variety of monomers, is tolerant of trace impurities (water, oxygen, inhibitor), and is readily applicable to industrial processes. The system that was developed was termed Atom Transfer Radical Polymerization (ATRP). ATRP is a robust system that has generated much interest among polymer chemists in both industry and academia. Science Watch, a trade journal, has recently listed three ATRP papers among the top ten cited papers in chemistry today.
The control of the polymerization afforded by ATRP is a result of the formation of radicals that can grow, but are reversibly deactivated to form dormant species. Reactivation of the dormant species allows for the polymer chains to grow again, only to be deactivated later. Such a process results in a polymer chain that slowly, but steadily, grows and has a well-defined end group (for ATRP that end group is usually an alkyl halide).
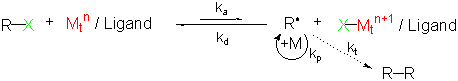
The initiator is generally a simple, commercially available, alkyl halide. The catalyst is a transition metal that is complexed by one or more ligands; the catalyst does not need to be used in a one-to-one ratio with the initiator but can be used in much smaller amounts. The deactivator can be formed in situ, or for better control, a small amount (relative to the catalyst) can be added. Additionally, the catalyst is tolerant of water and trace amounts of oxygen.
Although other controlled radical polymerization systems have been reported by various groups, ATRP remains the most powerful, versatile, simple, and inexpensive. Only ATRP has been able to polymerize a wide range of monomers including various styrenes, acrylates and methacrylates as well as other monomers such as acrylonitrile, vinyl pyridine, and dienes. ATRP commonly uses simple alkyl halides as initiators and simple transition metals (iron, copper) as the catalysts. These catalysts can be used in very low amounts, whereas, other controlled polymerization systems require the use of expensive reagents in much higher concentrations.